Fossil Alpha-Recoil Analysis of Certain Variant Radioactive Halos
Science, vol. 160, pp. 1228-1230, 1968.
Abstract. The distribution of alpha-radioactivity in the vicinity of uranium and of certain variant radioactive halos in biotite was investigated by the fossil
alpha-recoil method. Within the limits of the method I could not confirm a previously proposed hydrothermal mechanism for the origin of certain variant halo
types due to polonium isotopes.
Microscopic examination of thin sections (≈ 20 μ) of certain minerals sometimes reveals a distinctive pattern of colored concentric rings
surrounding a minute central inclusion about 0.5 to 1 μ in radius. Although these structures had long been observed by mineralogists, their origin was a
mystery until almost simultaneously Joly (1) and Mugge (2) correctly attributed the phenomenon to the presence of radioactivity in the central inclusion. While
in some instances the inclusions have been identified as zircon (1,
3), xenotime, or monazite (4), the halo nuclei are often too small for petrologic analysis.
In polarized light, the appearance of the varicolored ring patterns in such anisotropic minerals as biotite suggested the designation "pleochroic halos," although
"radioactive halos" is clearly more appropriate. While the radioactivity in the central inclusion may consist of α-, β-, and γ-radiation, the
development of a halo is basically due only to the proportionately much higher ionization effects of the α-particles. This is an extremely fortuitous
situation because, since the α-particle has a rather precise range R in a mineral for a given initial energy E, one can often ascertain not only the elements
responsible for a particular halo type but also the specific isotopes. If the halo nucleus contains uranium, the α-emission from the eight α-emitters
in the decay chain produces a region of radiation damage surrounding the inclusion. In certain biotites this region becomes faintly visible when about 108 atoms
of 238U have decayed; with increased α-emission a series of colored, spherically concentric shells eventually appears, corresponding to the ranges of the
respective α-emitters of the 238U decay chain. The three-dimensional nature of the halo becomes strikingly apparent when a sample of biotite is prepared
for microscopy. The leaves of a book of mica are easily cleaved with transparent cellophane tape, and each successive layer of mica reveals a ring pattern of
increasing size until the diametral section is obtained. Years ago there was great interest in the ring structure of uranium and thorium halos in investigation of
the invariance of the radioactive transformation rate over geological time (5). It is in this connection that radioactive halos have again drawn interest (6).
Naturally ring sizes are always measured from diametral sections; results are best from specimens having exceptionally small nuclei. Use of a filar micrometer
shows the ring radii for the uranium and thorium halos to agree very well with the calculated α-particle ranges of 238U and 232Th and their
respective α-emitters. Thus an experimental range:energy relation for α-particles may be determined for any mineral containing well-defined
uranium or thorium halos, with small central inclusions.
Certain types of halos (I call them variant halos) exist that cannot be identified with the ring structure of either the uranium or thorium halos. What is the nature
of the α-emitters responsible for these variant halos? Several types of variant halos were discovered but were not claimed to be evidence of new
α-emitters because radioactive-decay schemes of uranium and thorium were still being refined. Nevertheless Joly (7) reported three variant halo types:
one he attributed to "emanation" (222Rn), a dwarf having a very small radius; another was simply designated the X-halo. Others (8-10) have reported unusual
halo sizes, and I have found halos having anomalous ring structure (11, 12). For greater clarification of the variant halos, I classify as class I those rather easily
identifiable with known α-emitters; as class II, those (such as Joly's X-halo) whose ring structure has not been correlated with known α-emitters.
For example, Henderson reported four variant halo types: A, B, C, and D. Types A, B, and C were correctly attributed to the polonium isotopes 210Po, 214Po, and
218Po, respectively; thus they are of class I. But I have been unable to confirm Henderson's association of the D-halo with 226Ra (13). I confine this report to
investigation of class-I halos—in particular to analysis of Henderson's proposed origin of the polonium halos.
The polonium isotopes have relatively short half-lives; any mechanism proposed for their origin must be consistent with this fact. The 218Po halo (Fig. 1, left),
so-called because 218Po is the initiating isotope, exhibits three rings arising from successive α-decay of 218Po (E1, 6.0 Mev; r1, 23 μ), 214Po (E2, 7.68
Mev; r2, 34 μ), and 210Po (E3, 5.3 Mev; r3, 19 μ). Ei and ri denote, respectively, the α-particle kinetic energy and the corresponding average
halo-ring radius. By analogy the 214Po and 210Po halos (Fig. 1, right) are, respectively, dual and single ring patterns. I have observed the polonium halos in many
Precambrian biotites, and the halos in Fig. 1 were found in biotites from the Baltic (Norway) and Canadian shields, respectively. Since these polonium isotopes
are daughter products of 238U, it was initially conceived (10) that they were preferentially fixed out of uranium-bearing solutions at localized deposition centers
along small conduits or veins within the host mineral (mica, for example).
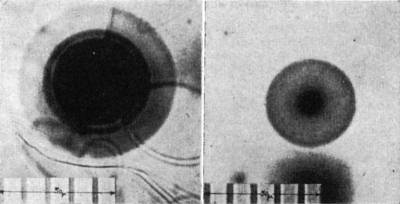
Fig. 1. Halos of 218Po (left) and 210Po (right).
|
While coloration surrounding minute veins in the mica is an indication of the flow of radioactive solutions (very weak solutions may show no staining
whatsoever), it does not follow that halos that formed around small nuclei in the conduits were necessarily derived from radioactivity in solution. For example,
polonium, uranium, and thorium halos also form around very small inclusions, with no visible conduit or crack in the mica connecting the halo nuclei, and it is
certainly not clear that these halos are of hydrothermal origin.
An attempt to determine whether the halo nuclei were capable of acting as selective fixation sites for certain radionuclides, by electron-microprobe analysis of
the halo inclusions, failed because of the small size involved. However, refinement of techniques may lead [p. 224] to clarification of the nature of the inclusions (14).
Thus a more sensitive technique is required for testing of the hypothesis regarding genesis of the polonium halos from a uranium-bearing solution.
Fission-track techniques (15) may serve this purpose. Uranium-238 fissions spontaneously, and the damaged regions in the host mineral, produced by the
fission fragments, can be enlarged sufficiently by acid etching for visibility under an optical microscope. Immersion of biotite samples, containing the polonium
and uranium halos in hydrofluoric acid for a few seconds and subsequent observation of the areas in the vicinity of the inclusions reveal a striking difference:
the polonium halos are characterized by complete absence of fission tracks, whereas the uranium halos always show clusters of fission tracks.
To eliminate the possibility that fission tracks may have been annealed out of the sample, I have irradiated mica specimens containing the uranium and
polonium halos with a neutron flux of 5 × 1017 neutrons per square centimeter and again etched the mica. The uranium halos show, as expected, marked
increase in the number of fission tracks emanating from the central inclusion, due to neutron-induced 235U fission, whereas the polonium halos are again
completely devoid of tracks (12).
If a uranium solution had been in a conduit feeding the central inclusions of the polonium halos with daughter-product activity, about 70 fission tracks per
centimeter of conduit would be expected by use of Henderson's model (10). This result depends on such parameters as the uranium concentration in the
solution, the rate of flow (conservatively I have assumed that the solution ceased to flow when the polonium halos formed), and the total number of polonium
atoms (5 × 108) necessary to form a well-developed 218Po halo. This last value I determined by observing the degree of coloration in uranium halos as a
function of the number of fission tracks emanating from the halo nucleus, the total number of α-particles required for production of a halo being
computed as eight times the number of fission tracks times the ratio of the half-lives for spontaneous fission and alpha decay for 238U. While fission tracks are
observed along stained conduits, in general I cannot correlate the distribution of fission tracks along clear conduits with the presence of polonium halos.
Polonium halos are also found randomly distributed throughout the interior of large mica crystals far removed from any conduit. (A limited survey may indicate
halos occurring within certain cleavage planes, but more extensive search shows this is not the case.) The question now arises of whether the source of the
short-half-life radioactivity, characteristic of such polonium halos, was due to (i) the laminar flow of a non-uranium-bearing solution, containing disequilibrium
amounts of daughter-product α-activity, through a thin cleft parallel to the cleavage plane, or (ii) the diffusion of gaseous radon through the mica. The
latter case has been considered (8), but only recently has the discovery of α-recoil tracks in micas (16) enabled quantitative checking of either of these
mechanisms. This technique is based on the fact that an atom recoiling from α-emission impinges on the host mineral and forms a damaged region large
enough to produce a pit which is visible in phase contrast when etched with hydrofluoric acid.
The original experiment (16) determined that a series of multiple recoils, such as is expected in the sequential α-decay of 238U and 232Th, yields
α-recoil tracks. Two additional points necessary for a complete α-recoil analysis—(i) whether a single α-recoil produces a track, and
(ii) whether α-recoil pits form in a sample placed in contact with an α-emitter—have now been resolved.
Several samples of mica were annealed for removal of background α-recoil pits; three different concentrations of dilute solutions of americium (5
percent 241Am and 95 percent 243Am) were evaporated on separate samples, and an α-count was taken. The daughter products of the americium isotopes
have very long half-lives, so that any α-recoil pits occurring reflect only single α-decay. The higher α-count samples yielded correspondingly
higher α-recoil densities within the area of deposition, accompanied by almost complete absence of tracks outside the radioactive zone. Thus was
established the existence of one α-track from a single α-recoil (17).
Corresponding α-recoil densities were also noted in annealed mica samples placed in contact with the americium-coated samples. It follows that any
excessα-radioactivity in micas may be effectively determined by analysis of the samples by the α-recoil technique.
The procedure for ascertaining the extent of increased α-activity consists in measuring background fossil α-recoil track densities in areas far
removed from the halos themselves, and in comparing these values with the densities near the halos for determination of the degree of excess α-activity.
Samples of Precambrian mica from Canada and Ireland (18), containing uranium and polonium halos, were investigated by etching in 48 percent hydrofluoric
acid for about 15 to 50 seconds. As in earlier experiments, 238U halos revealed the presence of fission tracks emanating from the central inclusions, whereas no
fission tracks were noted from the central inclusions of the polonium halos.
The experimental procedure was to photograph in phase contrast a given etched area, enlarge, and count anywhere from several hundred to 1000 α-recoil
centers for each density measurement. The enlargement factor was determined by photographing the rulings of a stage micrometer, using each objective.
Replicate measurements were made on several areas with different [p. 225] halo types. The background fossil α-recoil density was measured before a count was
made in the mica cleavage plane about 5 to 10 μ directly above the halo nucleus. The mica was then cleaved until the central inclusion appeared on the
surface; the mica was etched again and another count was made to enable a density comparison of three separate regions.
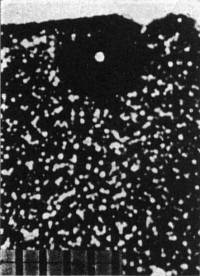
Fig. 2. Fossil α-recoil centers in the vicinity of a 210Po halo (phase contrast).
|
The mean fossil α-recoil densities were 12.7 × 106 and 11.6 × 106 α / cm2 for the Canadian and Irish micas, respectively, regardless of
where the α-recoil count was taken. For a given etch period these results are reproducible within ± 10 percent. The fission-track density exhibited a
random distribution in each piece of mica except (as expected) near the 238U halos. The α-recoil:fission-track ratios were about 2.5 × 103 and 3.0
× 103, respectively, for the Canadian and Irish micas. Huang and Walker (16) have shown that the background α-recoil density in micas is due to
both uranium and thorium α-decay; by using 100 Å and 10 μ for the alpha-recoil and fission-track ranges, respectively, one can determine
that uranium alone contributes an α-recoil:fission-track ratio of about 2.2 × l03, any excess being due to thorium. Figure 2 portrays a 210Po halo
(Irish mica) showing the distribution of α-radioactivity (fossil α-recoil centers) in the vicinity.
As far as the experimental analysis is concerned, there is no detectable difference in the microscopic distribution of α-radioactivity (with respect to
background density) near either the uranium or the polonium halos. [I note that thin clefts, which usually result near the edges of the mica from weathering (but
not within the bulk of the mica), are easily detected by an acid etch since α-recoil tracks appear throughout the extent of the cleft area.] This finding
seems to imply that there was no gross transport of α-radioactivity to the polonium-halo inclusions (i) by way of laminar flow of solutions (through thin
clefts) disequilibrated as to uranium daughter-product activity, or (ii) by diffusion of radon, since an increased α-recoil density, higher than background
by several orders of magnitude, should be evident within a l0-μ radius of the halo inclusions in either case. This last value is a conservative estimate, for I
have considered only the decay of 218Po atoms en route to an inclusion. Furthermore, autoradiographic experiments on the samples of Canadian mica containing
238U,232Th, and polonium halos showed only the normal background distribution of α-tracks, indicating that if excess activity now exists it is below the
detection level of the method.
Thus, as far as the experimental analysis is concerned, I cannot confirm Henderson's model for the secondary origin of the polonium halos. To the question of
what mode of origin is consistent with the relatively short half-lives of the polonium isotopes (or their β-decaying precursors), I can say only that other
mechanisms are under study.
Whatever hypothesis is invoked, to explain the origin of the polonium halos, must also explain both the one found by Henderson (19) [due to a combination of
isotopes from both the thorium series (212Po and 212Bi) and the uranium series (210Po)] and a halo presumably due to 211Bi (12) from the 235U series. Perhaps most
interesting of all is the occurrence of 20,000 to 30,000 218Po and 210Po halos per cubic centimeter in a Norwegian mica—without the 214Po halos.
| ROBERT V. GENTRY |
Institute of Planetary Science,
Columbia Union College,
Tacoma Park, Maryland 20012 |
References and Notes
- J. Joly, Phil. Mag. 13, 381 (1907).
- O. Mugge, Zentr. Mineral. 1907, 397 (1907) (see Oak Ridge National Laboratory ORNL-tr-757).
- J. Joly, Phil. Trans. Roy. Soc. London Ser. A 217, 51 (1917); P. Ramdohr, Geol. Rundschau 49, 253 (1960) (see ORNL-tr-758).
- C. O. Hutton, Amer. J. Sci. 245, 154 (1947).
- J. Joly, Nature 109, 480 (1922); F. Lotze, ibid. 121, 90 (1928).
- G. Gamow. Phys. Rev. Letters 19, 759 (1967).
- J. Joly, Proc. Roy. Soc. London Ser. A 102, 682 (1923).
- S. Iimori and J. Yoshimura, Sci. Papers Inst. Phys. Chem. Res. 5, 11 (1926); A. Schilling, Neues Jahrb. Mineral. Abhandl. 53A, 241 (1926) (see ORNL-tr-697).
- J. S. van der Lingen, Zentr. Mineral. Abt. A 1926, 177 (1926) (see ORNL-tr-699); C. Mahadevan, Indian J. Phys. 1, 445 (1927); H. Hirschi, Vierteljahresschr.
Naturforsch. Ges. Zuerich 65, 209 (1920) (see ORNL-tr-702); E. Wiman, Bull. Geol. Inst. Univ. Uppsala 23, 1 (1930); G. H. Henderson, Proc. Roy. Soc.
London Ser. A 173, 238 (1939).
- G. H. Henderson, Proc. Roy. Soc. London Ser. A 173, 250 (1939).
- R. V. Gentry, Appl. Phys. Letters 8, 65 (1966); Earth Planetary Sci. Letters 1, 453 (1966).
- ——, Nature 213, 487 (1967).
- Observations on this and other class-II halos will be reported.
- I thank Larry Kobren, Goddard Space Flight Center, for the electron-microprobe analysis. Also I thank Truman Kohman, Carnegie-Mellon University, for
suggesting the micro-probe experiments and for valuable discussions concerning the origin of the halos.
- R. L. Fleischer, P. B. Price, R. M. Walker, Science 149, 383 (1965).
- W. H. Huang and R. M. Walker, ibid. 155, 1103 (1967).
- J. Boyle and R. V. Gentry, in preparation.
- G. H. Henderson, Proc. Roy. Soc. London Ser. A 145, 591 (1934).
- I thank G. C. Milligan and other members of the geology and physics departments of Dalhousie University, Halifax, for the loan of Henderson's halos and
microphotographs. The halo referred to is in this collection.
- I thank Paul Ramdohr, University of Heidelberg, for this particular specimen. Also I thank R. R. Gorbatschev (Uppsala), B. Loberg (Stockholm), D. E.
Kerr-Lawson (Swastika, Ontario), J. H. J. Poole (Trinity College), and J. A. Mandarino (Royal Ontario Museum) for other mica specimens containing halos. I
also thank H. L. Price for assisting in the α-recoil analysis and John Boyle. Oak Ridge National Laboratory, for the α-recoil experiments. For more
extensive investigation I would appreciate contributions of samples of biotite from as many Precambrian localities as possible.
26 April 1968
|
![Logo shows magnified cross-section of a Polonium 218 halo in a granite rock. How did it get there? [halos.com]](../logo.jpg)