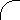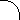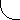|
Chapter 2: The Genesis Rocks
[p. 26]
Extinct Halos Intrude on the Scene
For a while it seemed the experiments on the D halos were done for no good purpose. In retrospect, it appears
they were the most important experiments I could have done at the time. They successfully focused my attention on
the A, B, and C halos. Without that focus it is quite possible that my research would soon have ceased. For over a
year I had dismissed the A, B, and C halos as being unimportant, not worthy of investigation. Outwardly it seemed
that the autoradiographic experiments hadn't shown anything startling at all. In contrast to the uranium and D
halos, there was, with one possible exception, a complete absence of alpha tracks from the A, B, and C halos after
the emulsion was developed. But it was this general nothingness that finally attracted my attention; it occurred to
me that the radioactivity that produced these halos really was extinct! I remembered that Henderson had described
these halos in considerable detail and had discussed extinct radioactivity in connection with them. I now went back
and carefully reviewed his evaluation.
My measurements of the various halo ring sizes confirmed his tentative conclusion that the A, B, and C halos
had originated with alpha radioactivity from three isotopes of the element polonium. These three
isotopes—polonium-210, polonium-214 and polonium-218 (in scientific notation 210Po, 214Po and 218Po)—are all
members of the uranium decay chain. This didn't necessarily mean that the 210Po, 214Po and 218Po halos were
generated by polonium atoms derived from uranium, but for reasons to be discussed shortly, Henderson postulated
that this was the case. He theorized that, sometime in the past, solutions containing uranium and all its daughters
must have flowed through tiny cracks, cleavages or conduits in the mica. Under these special conditions he
proposed that the isotopes necessary to produce the different polonium halos would gradually accumulate at certain
points along the path of the solution. Supposedly, after a certain time, a sufficient number of atoms would be
collected for a polonium halo to form.
Earlier this explanation had seemed so plausible that I promptly accepted it and almost lost interest in the A, B,
and C halos. However, since the emulsion experiments had shown that their radioactivity was extinct, I became
quite interested in why they were extinct and began to think more critically about Henderson's proposed mode of
origin. Figures 2.1-2.3 show idealized three-dimensional views of the 210Po, 214Po and 218Po halos. (Color photos of
these halos appear in the Radiohalo Catalogue.)
[p. 27]
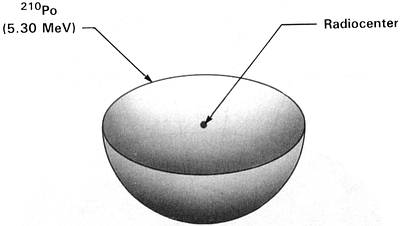
(210Po half-life = 138.4 days)
(210Pb half-life = 22 years)
Figure 2.1 210Po Halo Cross Section
Idealized three-dimensional illustration of a 210Po halo obtained by slicing the halo through the center.
Each halo ring is identified by the appropriate isotope and its alpha energy in MeV (Million electron
Volts).
|
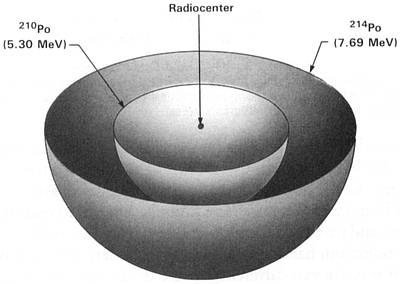
(214Po half-life = 164 microseconds)
(214Pb half-life = 26.8 minutes)
Figure 2.2 214Po Halo Cross Section
Idealized three-dimensional illustration of a 214Po halo obtained by slicing the halo through the center.
Each halo ring is identified by the appropriate isotope and its alpha energy in MeV (Million electron
Volts).
|
[p. 28]
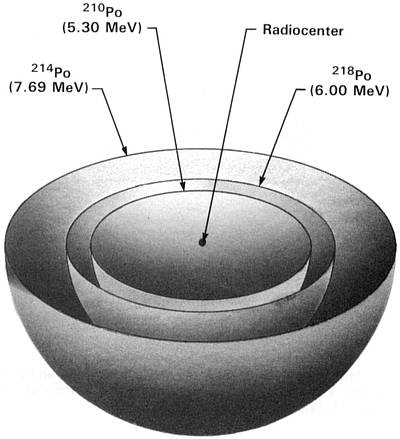
(218Po half-life = 3 minutes)
Figure 2.3 218Po Halo Cross Section
Idealized three-dimensional illustration of a 218Po halo obtained by slicing the halo through the center.
Each halo ring is identified by the appropriate isotope and its alpha energy in MeV (Million electron
Volts).
|
Could Henderson's hypothesis for the secondary origin of polonium halos be tested? He had suggested this
should be done. His entrance into Canadian defense work during World War II and his death soon afterward
prevented him from doing the tests himself. I began to examine polonium halos closely and paid special attention to
why Henderson felt it was necessary to explain polonium halos by some sort of secondary mechanism. Of course!
The reason was the vast difference in the decay rate, or average life span, between the uranium atoms and the
polonium atoms. Any hypothesis proposed for the origin of the polonium halos had to take this difference into
account. On the average, uranium atoms are now decaying so slowly that it would take 4.5 billion years for half of
them to undergo radioactive decay. In contrast, the three isotopes responsible for the origin of the polonium halos,
namely 210Po, 214Po and 218Po, decay far more rapidly. Their brief [p. 29] life spans present some unique problems in
formulating a satisfactory hypothesis for the origin of the respective halos.
The following synopsis, showing what types of radioactivity fit into evolutionary model of the origin of our
planet, will enable the reader to more readily grasp the significance of these problems.
Modern Cosmology and Extinct Natural Radioactivity
According to the evolutionary Big Bang scenario, our planet began as a hot molten sphere several billion years
ago. Cosmologists admit that the Big Bang, if it occurred, could have produced only hydrogen (H) and helium (He)
and that the earliest stars were composed of just these two lightest elements. They assume the heavier chemical
elements, of which the earth is mostly comprised, originated in thermonuclear reactions (nucleosynthesis) in the hot
interiors of various stars. Supposedly these elements were ejected into space when these stars later exploded as
supernovae. They further believe that the newly synthesized elements from one or more supernovae eventually
reaccumulated to form vast interstellar clouds of gas. It is assumed that one of these clouds later condensed to form
the primeval sun and then the embryonic planets of our solar system. Cosmologists believe a long time elapsed
between nucleosynthesis and the formation of the primordial earth. And they also think a certain kind of
radioactivity can reveal the approximate length of this period.
In particular, they envision that some radioactive elements formed at nucleosynthesis decayed so slowly that
significant fractions of the original amounts have survived to the present—uranium and thorium are
examples. They also believe, however, there were other elements whose decay was slow enough for them to be
initially incorporated into the primordial earth, but which have almost completely decayed away during the last
several billion years. Extinct natural radioactivity is the term used for this special category of radioactive elements.
Scientists have diligently sought for extinct natural radioactivity in various rocks because they think it could
provide an upper limit on the time interval between nucleosynthesis and the formation of the primordial earth.
Because they believe this interval was tens or hundreds of millions of years long, they have searched for certain
long half-life (tens of millions of years) radioisotopes in Earth's crustal rocks. One isotope of plutonium (not to be
confused with polonium) with a half-life of 83 million years has been found and accepted as extinct natural
radioactivity because it fits in with the Big Bang scenario. (Modern cosmologists would [p. 30] consider it useless
to look for decay products of relatively short half-life radioactivity, for in their view it would be impossible for
there to be such evidence of extinct natural radioactivity.)
|
![Logo shows magnified cross-section of a Polonium 218 halo in a granite rock. How did it get there? [halos.com]](../logo.jpg)