Chapter 1: Radiohalos and the Age of the Earth
The Radioactive Nature of the Halos
In 1907 the solution to the halo puzzle came into focus in the geology laboratory of Professor John Joly of
Trinity College in Dublin. Joly was quite familiar with halos, especially those in biotite, a dark mica that is easily
split into thin slices. Joly realized the diffusion hypothesis could not explain either the well-defined edges of the
halo rings, or their regular sizes. He began to consider a radioactive origin for the halos.
By that time scientists knew that uranium is the parent of a radioactive decay chain, with the successive
daughter products being called the members of the chain. This decay chain is shown in Figure 1.1 along with other
relevant information. Joly was also aware that uranium and its radioactive daughter products decayed in one of two
ways: (1) By ejecting a very light fragment (the beta particle), which causes little damage as it passes through
matter, or (2) by ejecting a much heavier nuclear fragment (the alpha particle), which interacts rather strongly as it
passes through a substance. Because of its light weight the beta particle is easily bounced around and thereby takes
a rather unpredictable, zigzag path before it finally comes to rest in matter. The alpha particle, on the other hand, is
heavy enough to plow almost straight ahead before it stops.
As Joly thought about which of these particles might be responsible for the halos, doubtless he quickly realized
that the light beta particles would be unlikely to produce coloration changes in the mica, and that their zigzag paths
could not yield sharp boundaries. The heavier alpha particles seemed much more promising candidates. Studies had
shown that most alpha emitters in the uranium decay chain had different energies, with the isotope 238U being the
lowest. (See Fig. 1.1 for further explanation.)
Was there a connection between the different energies of those alpha particles and the different sizes of the
rings in the halos Joly had observed? Alpha particles having different energies would travel slightly different
distances in a mineral. What if some uranium was in the tiny halo center? Could alpha particles from uranium and
its daughters cause enough damage to the surrounding mineral to produce the pleochroic halos?
In a mineral, alpha particles lose their energy quite rapidly through collisions with other atoms. A single alpha
particle will ionize about 100,000 atoms along its path of travel, leaving in its wake a short damage trail which
remains as a permanent scar. On an atomic scale the damage to the mineral is so small that by itself each tiny scar
is invisible. Any mineral, such as mica, which contains trace amounts of uranium, will also contain some
[p. 19] alpha-damage trails from the uranium atoms that have already decayed. Generally, however, the
uranium atoms are uniformly dispersed throughout the mineral so that the damage trails do not overlap. Thus, a
mineral may be filled with invisible alpha-damage trails. Even in the instances when several uranium atoms, or
even several hundred, are close enough to produce overlapping trails, this amount of overlap is still insufficient to
produce noticeable color changes in the mineral.
|
[p. 18] Figure 1.1 Glossary of Technical Terms
Radioactive atoms are capable of spontaneously changing, or decaying, to atoms of a different type. A
parent radioactive atom decays into a daughter atom in various ways, one of which is by
the emission of an alpha (α) particle. Numerous types of radioactive atoms occur in nature, but only
three are the initiators of a decay chain. For this book the one beginning with uranium-238 (238U in
scientific notation) is most important.
The numerical superscript denotes the number of protons and neutrons in the nucleus and signifies how
heavy the element is. Isotopes of the same element have different masses but nearly identical chemical
behavior—as for example (238U and 235U). An alpha (α) particle has a mass of 4.
Uranium-238 initiates a chain of steps which ends in the element lead (chemical symbol Pb). The 238U decay
chain, as shown below, has some daughters which decay by emitting a beta (β) particle, which is nearly 7400
times lighter than the more massive alpha (α) particle. The type of decay is shown by the symbols α
and β.
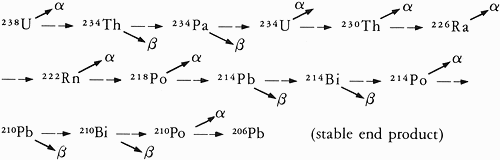
U-uranium
Th-thorium
Pa-protactinium
Ra-radium |
|
Rn-radon
Po-polonium
Bi-bismuth
Pb-lead
|
|
The half-life of a radioactive isotope is the time required for half the atoms in any collection to decay.
If 1000 atoms exist at a certain time, then only 500 will remain after one half-life, after two half-lives only 250
atoms of the original collection will remain, and so forth. Half-life and decay rate are closely
related quantities. isotopes that decay quickly have short half-lives; those that decay more slowly have longer half-
lives. At present 238U is decaying very slowly with a half-life of 4.5 billion years.
|
In contrast, imagine billions of uranium atoms clustered in the tiny grain at a halo center. Alpha particles
ejected from this grain can be compared to the appearance of a vast array of needles stuck into a point. To Joly it
seemed quite plausible that the overlapping damage effects of this sunburst pattern of alpha particles might just be
sufficient to produce the coloration seen in a halo. Figure 1.2 illustrates this effect.
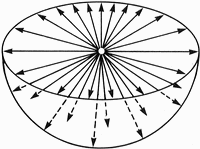
| Figure 1.2 Sunburst Effect of Alpha-Damage Trails |
Sunburst pattern of alpha-damage trails produces a spherically colored shell around the halo center.
Each arrow represents 5 million alpha particles emitted from the center. Halo coloration initially develops
after 100 million alpha decays, becomes darker after 500 million, and very dark after 1 billion.
|
Only one main question now remained: Did the sizes of the halo rings correspond to the path lengths of the
uranium series alpha particles in mica? Measurements had shown alpha particles from the uranium decay chain
traveled from about three to seven centimeters in air before coming to rest. Joly calculated that in mica alpha
particles travel only 1/2000 as far as in air. Reducing the measured air-path lengths of the uranium series alpha
particles by this factor gave values which did correspond to the ring sizes of one halo type he had found. The pieces
of the puzzle had fallen into place. Joly proposed that alpha emission from the tiny halo center could account for
both the sphericity and the size of the different shells comprising the halos. Moreover, the fact that alpha particles
do most damage near the end of their paths would explain why the outer edges of halo rings could [p. 20] be darker
than the interior regions. Thus Joly specifically identified uranium and a companion element, thorium, as
radioactive elements that could produce pleochroic halos. Quite appropriately, they later became known as
radioactive halos, or radiohalos.
Figure 1.3 graphically illustrates the idealized three-dimensional cross section of a uranium halo. Color photos
of uranium halos appear in the Radiohalo Catalogue. Those photos show five rings of the uranium halo; these can
be accounted for by the eight alpha emitters in the uranium decay chain as shown in Fig. 1.3. Figure 1.1 shows
there are also six beta emitters in this chain, but, as just discussed, their interaction with mica is insufficient to
produce halo rings.
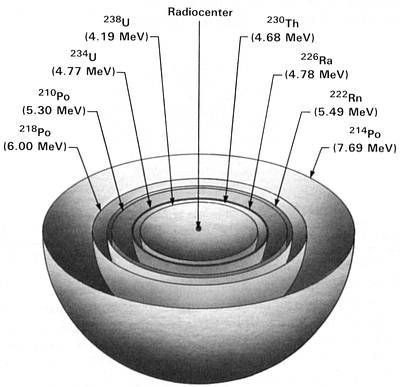
238U Halo Cross Section
(238U half-life = 4.5 billion years)
Figure 1.3 Uranium Halo Cross Section
Idealized three-dimensional illustration of a uranium halo obtained by slicing the halo through the
center. Each halo ring is identified by the appropriate isotope and its alpha energy in MeV (Million electron
Volts).
|
|
![Logo shows magnified cross-section of a Polonium 218 halo in a granite rock. How did it get there? [halos.com]](../logo.jpg)